Acid test on productivity
 PRODUCTION ADVICE - JUNE 2021 - AGRONOMY
PRODUCTION ADVICE - JUNE 2021 - AGRONOMY
By Adrian Smith
Senior Land Services Officer - Mixed Farming Systems
P: 03 5881 9932 | M: 0447 778 515 | E: adrian.smith@lls.nsw.gov.au
Soil acidity continues to be one of the main soil-related challenges faced by producers in southern NSW.
While it has been a familiar issue for those on the slopes and in higher rainfall areas for a while, highly acidic soils are now becoming more commonplace on the plains, and in particular in the irrigation areas.
So, what is soil acidity?
Most would be aware that acidity is measured on a (pH) scale of 1-14, with anything below a pH of 7 considered acidic, and above a pH of 7 is alkaline, with 7 being neutral. Soil pH is a measure of the amount (or concentration) of hydrogen (H+) ions in the soil solution. The pH is measured either in a water solution or in a CaCl2 (Calcium Chloride) solution. Generally, the pH values when measured in water are 0.5-1.0 units higher than in CaCl2.
The other important thing to note about the pH scale is that it is logarithmic. Soil with a pH of 5 has 10 times more acid than soil with a pH of 6, and 100 times more acid than a soil with a pH of 7. This has some important implications when it comes to managing soil acidity.
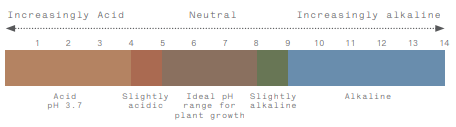
Most of our agricultural soils are in the pH 4-9 range, and most plants prefer a pH in the 5-8 range.
Why is soil pH important?
In broad terms, soil pH will tend to decrease (become more acidic) over time under modern agriculture systems.
The pH of the soil will be influenced by several factors, including the parent material, leaching of nutrients, product removal, breakdown of organic matter and use of synthetic fertilisers (particularly nitrogen-based fertilisers).
As plants grow, they excrete acid into the soil, and the soil becomes more acidic. The above-ground parts of the plant are generally alkaline, and when we harvest the plants (through grazing, harvesting grain, making hay etc) and remove the alkaline part of the plant, this further increases the acidity of the soil. Highly productive soils, where we are growing (and removing) a lot of plant material, tend to become more acidic quicker.
As soils become more acidic, we tend to see:
- decreased plant growth
- increased availability of aluminium (Al) - aluminium is not really required by plants, and there is a relationship where pH decreases (more acidic), Al becomes more available to plants and becomes toxic. Manganese can also become more toxic as pH falls.
- decreased nodulation of legumes (the rhizobium bacteria that fix nitrogen from the atmosphere into the soil are particularly impacted by acidic soils)
- possible increased leaching and/or reduced availability of ‘good’ nutrients such as magnesium, calcium, phosphorus and molybdenum
- plants tending to be more susceptible to attack by pests and diseases.

Effect of soil pH on growth of subterranean clover
What is the extent of the problem?
It is estimated that over half of the intensively farmed agricultural soils in NSW have some form of soil acidity problem.
However, even this sobering number may not be revealing its true extent.
Recent work conducted by NSW Department of Primary Industries (https://doi.org/10.1071/SR20079) suggests that our current (and widely accepted) approaches to soil sampling are in fact ‘masking’, or in some cases failing to detect, the extent of acidity.
Typically, most commercial soil sampling assesses topsoil to 10 cm. However, this new research suggests there is a high degree of ‘stratification’ of soil acidity, and that there may be a layer of higher soil acidity in the 5-10 cm layer. Simply measuring and aggregating a 0-10 cm sampling will ‘average out’ the acidity over the entire 10 cm and not specifically identify the real extent of any acid soil problem.
Further, by not sampling deeper than 10 cm, producers and their advisors are not identifying the depth and extent of any soil acidity problem in the deeper layers of their soil. After all, we want our plants to use and exploit as much of the soil profile as possible to make better use of stored soil water, nutrients etc. If we have no idea what the soil properties are at depth, can we be confident plants can take advantage of the moisture and nutrients down there?
Further, once acidification extends deeper into the subsoil, it becomes increasingly difficult (and costly) to ameliorate.
Managing acid soils
Typically, we apply lime to manage soil acidification.
Applying lime on the surface relies either on rainfall or irrigation to move it into the soil profile. However, the current research indicates that incorporating the lime into the soil profile through the use of discs as opposed to tined implements will be far more effective.
And not all limes are the same. Lime quality is measured by its ‘neutralising value’, which is a measure of its fineness. The finer the lime, the better the result.
While applying lime will have immediate impacts on reducing soil acidity, there is now plenty of evidence that shows the impacts of lime application will have long-term, lasting impacts on soil health. It appears it allows the soil to be more resilient, particularly in poorer seasons. Addressing soil pH appears to set the foundation for your production system over the longer term.
In the past, we have tended to apply lime once a problem has been identified. However, the current thinking is that if we can maintain our soils with a pH above 5.5 (CaCl2), then we are maintaining productivity and minimising the possibility of the acidity problem moving into the subsoil. It may mean more frequent (but smaller) lime applications, but the evidence suggests this will be far less costly over the long term.
Best strategies to manage soil acidification
- You can’t manage what you don’t measure – sample your soils as part of a regular program and measure separately the 0-5 cm, 5-10 cm and even the 10-15 and 15-20 cm layers to get a true indication of the extent of acidification in your soil.
- Maintain soil pH above 5.5 (CaCl2) through regular application of lime.
- Incorporation of lime will have both immediate and long-term benefits.
- Addressing soil acidification has multiple benefits to your farming business, appears to provide greater resilience, and provides far more options for production.
- Addition of organic matter, or increasing soil organic matter (which is low in nitrogen) can have a beneficial effect by increasing the buffering capacity (the soil’s capacity to resist pH change) of your soil.
- Manage nitrogen fertiliser inputs to better match plant needs to reduce nitrate leaching.
- There may be some other amendments for treating soil acidification – but lime remains at this stage the most cost-effective method to treat (and maintain) soil acidity.